81%
(n=70/86)
BLINCYTO® (blinatumomab) is indicated for the treatment of CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL) in first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1% in adult and pediatric patients. ... Read More
Study design: N=86, an open-label, single-arm phase 2 study of adult patients with MRD(+) B-cell precursor ALL who had received at least 3 chemotherapy blocks of standard ALL therapy, were in hematologic complete remission (defined as < 5% blasts in bone marrow, absolute neutrophil count > 1 Gi/L, platelets > 100 Gi/L), and had MRD* at a level of ≥ 0.1% using an assay with a minimum sensitivity of 0.01%. Primary endpoint: 81% (n=70/86) of patients had no detectable MRD assessed after 1 treatment cycle with BLINCYTO®.* Select secondary endpoints: OS, hematologic RFS at 18 months, duration of hematologic remission.1,2
*Defined as the absence of detectable MRD confirmed in an assay with minimum sensitivity of 0.01% for 6 patients and ≤ 0.005% for 80 patients. Undetectable MRD was achieved by 65 of 80 patients with an assay sensitivity of at least 0.005%.1
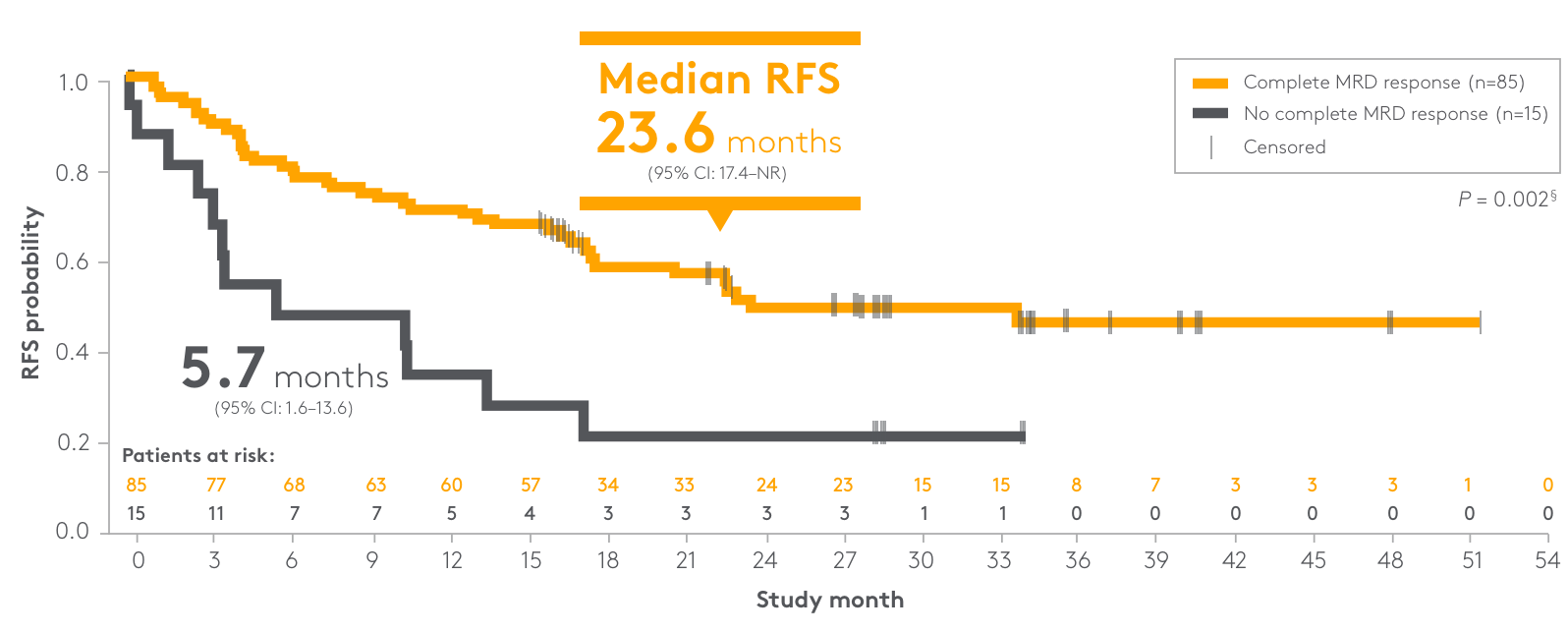
Primary endpoint: complete MRD response*
81%
(n=70/86)
of patients had no detectable MRD†
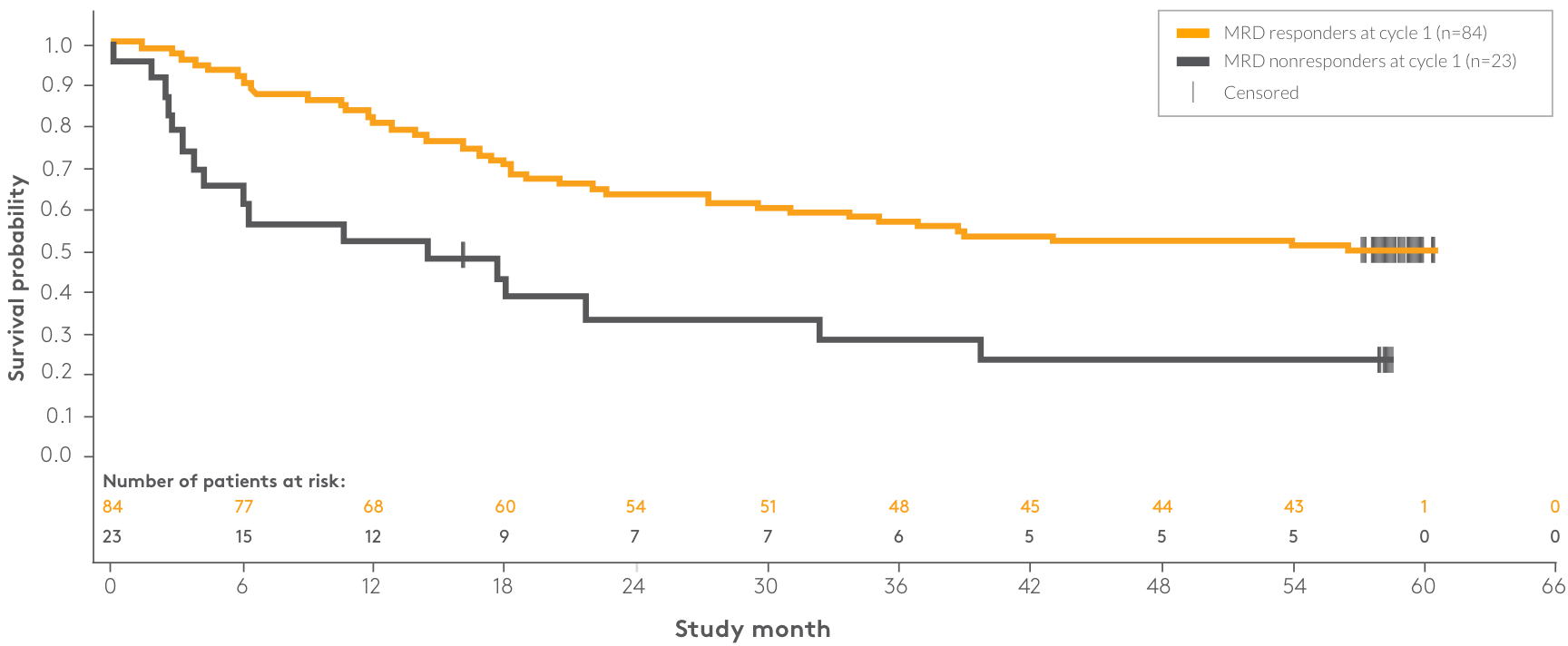

| Patient response | n | Median OS | 95% CI |
| MRD responders at cycle 1 | 84 | NR | 29.5–NR |
| MRD nonresponders at cycle 1 | 23 | 14.4 months | 3.8–32.3 |
Adults ≥ 18 years of age with B‑cell precursor ALL in hematologic first complete remission or second complete remission with MRD ≥ 0.1%*
Follow-up:
30 days
(safety);
24 months
plus
5-year survival follow-up
| Key inclusion criteria1 |
|---|
| Adults ≥ 18 years of age with B-cell precursor ALL in hematologic complete remission |
| Measurable residual disease (MRD)‡ level of ≥ 0.1% (molecular relapse or molecular failure)§ |
| < 5% blasts in bone marrow |
| ANC > 1 Gi/L |
| Platelets > 100 Gi/L |
| Key exclusion criteria4 |
| Prior HSCT |
| Presence of circulating blasts or current extramedullary disease |
| History of relevant CNS pathology or current relevant CNS pathology |
| Prior systemic cancer chemotherapy within 2 weeks or radiotherapy within 4 weeks |
| Baseline characteristics of patients (N=86) | |
|---|---|
| Age | |
| Median, years (min, max) | 43 (18, 76) |
| ≥ 65 years, n (%) | 10 (12) |
| Males, n (%) | 50 (58) |
| Philadelphia chromosome disease status, n (%) | |
| Positive | 1 (1) |
| Negative | 85 (99) |
| Relapse history, n (%) | |
| In first complete remission | 61 (71) |
| In second complete remission | 25 (29) |
| Baseline MRD levels,* n (%) | |
| ≥ 10% | 7 (8) |
| ≥ 1% and < 10% | 34 (40) |
| ≥ 0.1% and < 1% | 45 (52) |
Blinatumomab (BLINCYTO®) is the only NCCN Guidelines—recommended treatment option in consolidation for adult patients with Ph(–) B-cell precursor ALL who have persistent or rising MRD; the NCCN Guidelines recommend “therapy aimed at eliminating MRD prior to allogeneic HCT,” when possible.7
Study design: A large (N=405), international, randomized, controlled, phase 3 study of single-agent BLINCYTO® vs SOC chemotherapy in patients ≥ 18 years of age: refractory to primary induction therapy or to last therapy, in first relapse (first remission duration < 12 months), in second or later relapse, or in any relapse after HSCT. Primary endpoint was mOS: 7.7 months for BLINCYTO® (n=271) vs 4.0 months for SOC chemotherapy (n=134); P = 0.012; HR: 0.71 (95% Cl: 0.55–0.93).1 Selected secondary endpoints: CR within 12 weeks after initiation of treatment, CR/CRh*/CRi within 12 weeks after initiation of treatment, MRD remission rate, duration of remission, adverse event rates.1,8
BLINCYTO® is an established chemo-minimizing approach that significantly improved survival vs SOC chemotherapy1
*A censored subject is indicated by a vertical bar.1
ALL, acute lymphoblastic leukemia; CI, confidence interval; CR, complete remission; DOR, duration of response; HR, hazard ratio; HCT, hematopoietic cell transplantation; HSCT, allogeneic hematopoietic stem cell transplantation; mOS, median overall survival; MRD, measurable or minimal residual disease; NCCN, National Comprehensive Cancer Network; OS, overall survival; Ph(–), Philadelphia chromosome-negative; QoL, quality of life; R/R, relapsed or refractory; SOC, standard-of-care.
*OS in patients treated in first salvage was a prespecified subgroup analysis in TOWER; however, the OS efficacy in this subgroup was not a study objective and the study was not powered to assess OS efficacy in this subgroup.10
†A censored subject is indicated by a vertical bar.9
NR, not reached.
*OS in patients censored for allogeneic transplant was a prespecified sensitivity subgroup analysis in TOWER; however, the OS efficacy in this subgroup was not a study objective, and the study was not powered to assess OS efficacy in this subgroup.10
CR/CRh*/
CRi rates of
44%
(n=119/271)
(95% CI: 37.9–50.0)
for patients treated with BLINCYTO® vs 25% (n=33/134) (95% CI: 17.6–32.8) for patients treated with SOC chemotherapy8 (P < 0.001)
Median duration of remission for patients who achieved CR/CRh*/CRi8
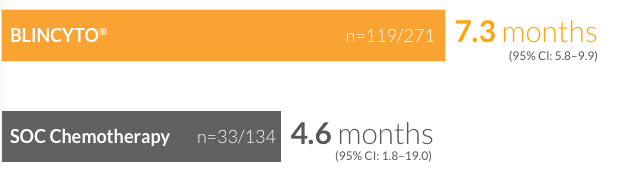
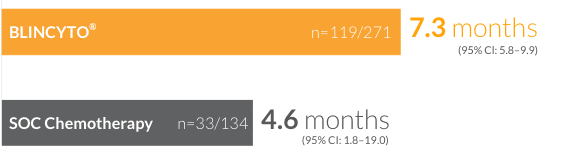
†CR was defined as ≤ 5% blasts in the BM, no evidence of disease, and full recovery of peripheral blood counts (platelets > 100,000/microliter and ANC > 1,000/microliter). CRh* was defined as ≤ 5% blasts in the BM, no evidence of disease, and partial recovery of peripheral blood counts (platelets > 50,000/microliter and ANC > 500/microliter). CRi was defined as ≤ 5% blasts in the BM, no evidence of disease, and incomplete recovery of peripheral blood counts (platelets > 100,000/microliter or ANC > 1,000/microliter).8
ANC, absolute neutrophil count; BM, bone marrow; CRh*, complete remission with partial hematologic recovery; CRi, complete remission with incomplete hematologic recovery.
74%
(n=20/27)
of patients achieved a best response of complete remission during continued therapy
BLINCYTO®
76%
(n=90/119)
vs SOC chemotherapy (n=16/33): 48%
†Molecular remission was assessed in patients achieving CR/CRh*/CRi, and was defined as MRD by PCR or flow cytometry with a minimum assay sensitivity of < 1 x 10–4.8
PCR, polymerase chain reaction.
Mean changes in HRQoL functional domains and symptoms at the end of Cycle 1 (Day 29)12
Click here for the EORTC QoL Questionnaire from the TOWER Study15
*The PRO instrument—The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30)—is a validated self-rating questionnaire used to assess patients’ perceptions of treatment effectiveness in oncology.13
AE, adverse event; HRQoL, health-related quality of life; PRO, patient-reported outcome.
Prephase treatment:
Premedication:
Primary endpoint:8
Selected secondary endpoints:8
| Key inclusion criteria 1,8 |
|---|
| Patients ≥ 18 years of age |
| Ph(–) R/R B-cell precursor ALL occurring as refractory to primary induction therapy or to last therapy, or untreated first relapse (first remission duration < 12 months), or in second or later relapse, or in any relapse after HSCT |
| With > 5% blasts in the BM and ECOG PS ≤ 2 |
| Key exclusion criteria 8 |
| Other active cancers |
| Clinically relevant pathologic condition of the CNS |
| Isolated extramedullary disease |
| Autoimmune disease |
| Acute GvHD of Grade ≥ 2, active chronic GvHD |
| Allogeneic stem cell transplantation within 12 weeks before randomization |
| Autologous stem cell transplantation within 6 weeks before randomization |
| Chemotherapy or radiotherapy within 2 weeks before randomization |
| Use of immunotherapy within 4 weeks before randomization |
| Ongoing use of investigational treatment |
BLINCYTO® was studied in a wide range of adult patients, including those with a poor prognosis8
|
BLINCYTO® (N=271) |
SOC Chemotherapy (N=134) | |
| Age | ||
| Mean ± SD, years | 41 ± 17 | 41 ± 17 |
| Range, years | 18–80 | 18–78 |
| Study entry criteria, n (%) | ||
| Refractory to primary or salvage therapy | 115 (42) | 54 (40) |
| In early first relapse (CR1 duration < 12 months) | 76 (28) | 37 (28) |
| In untreated second or later relapse§ | 32 (12) | 16 (12) |
| Relapsed after HSCT§ | 46 (17) | 27 (20) |
| Not specified | 2 (1) | 0 |
| Prior salvage therapy, n (%) | 171 (63) | 70 (52) |
| Prior transplant, n (%) | ||
| Yes | 94 (35) | 46 (34) |
| No | 176 (65) | 87 (65) |
| Unknown | 1 (0) | 1 (1) |
| Disease burden, n (%) | ||
| ≥ 50% BM blasts | 201 (74) | 104 (78) |
Blinatumomab (BLINCYTO®) is an NCCN-recommended Category 1 therapy option for adult patients with Ph(–) R/R B-cell precursor ALL7
In a real-world evidence study, the observed efficacy of BLINCYTO® was reflective of clinical study data1,8,18,*
*Five patients who could not titrate to the full dose due to tolerance were included in the analysis.18
Ph(–), Philadelphia chromosome–negative; Ph(+), Philadelphia chromosome–positive; TKI, tyrosine kinase inhibitor.
Median OS18
9.2 months
(n=158)
*One patient did not have data available for the survival analysis and is not included in the survival analyses. However, note that this patient is included in the toxicity and response evaluations.18
†Estimated from the time of BLINCYTO® treatment initiation to death or lost to follow-up.18
CR/CRi
rates of
57%
(n=86/151)
for patients on
BLINCYTO®
Median DOR for patients who achieved CR/CRi18,‡
for patients treated with BLINCYTO®
(n=86)
Duration of response by tumor burden18


*The exact assessment timing was not specified for this endpoint.18
†Data were missing for 8 patients in this analysis.18
‡Adjusted for HSCT.18
MRD response
78%
(n=65/83)
for patients
in CR treated
with
BLINCYTO®
Proceeded to HSCT18
for all patients treated with BLINCYTO®
(n=71/159)
*The exact assessment timing was not specified for this endpoint.18
Baseline characteristics (n=159)18
| Age* | |
|---|---|
| Mean ± SD, years | 45 ± 17 |
| Range, years | 8–79 |
| Study entry criteria, n (%) | |
| Refractory to primary therapy | 41 (26.6) |
| < 18 months to first progression | 76 (49.4) |
| Cytogenetic abnormalities, n (%) | |
| No | 62 (39.0) |
| Yes | 97 (61.0) |
| ≥ 3 previous therapies, n (%) | 36 (22.8) |
| Prior transplant, n (%) | |
| Yes | 27 (17.0) |
| No | 132 (83.0) |
| BM blasts at initiation of BLINCYTO®, n (%) | |
| BM blasts ≥ 50%, n (%) | 57 (45.2) |
*Pediatric patients were included in this analysis.
Study design: BLINCYTO® single-agent immunotherapy was evaluated in an open-label, single-arm, multicenter phase 2 study (N=45) in adult patients with Ph(+) R/R B-cell precursor ALL who progressed after, or were intolerant to second- or later-generation TKI therapy and were intolerant or refractory to imatinib. Primary endpoint was CR/CRh* rate within the first 2 treatment cycles: 36% (n=16/45; 95% CI: 22–51).1,19 Selected secondary endpoints: MRD response rate during the first two cycles of treatment, RFS, OS, HSCT after BLINCYTO®-induced remission.1,19
BLINCYTO® is an effective treatment for Ph(+) B-cell precursor ALL1
Primary endpoint: CR/CRh* rate within the first 2 treatment cycles1,19

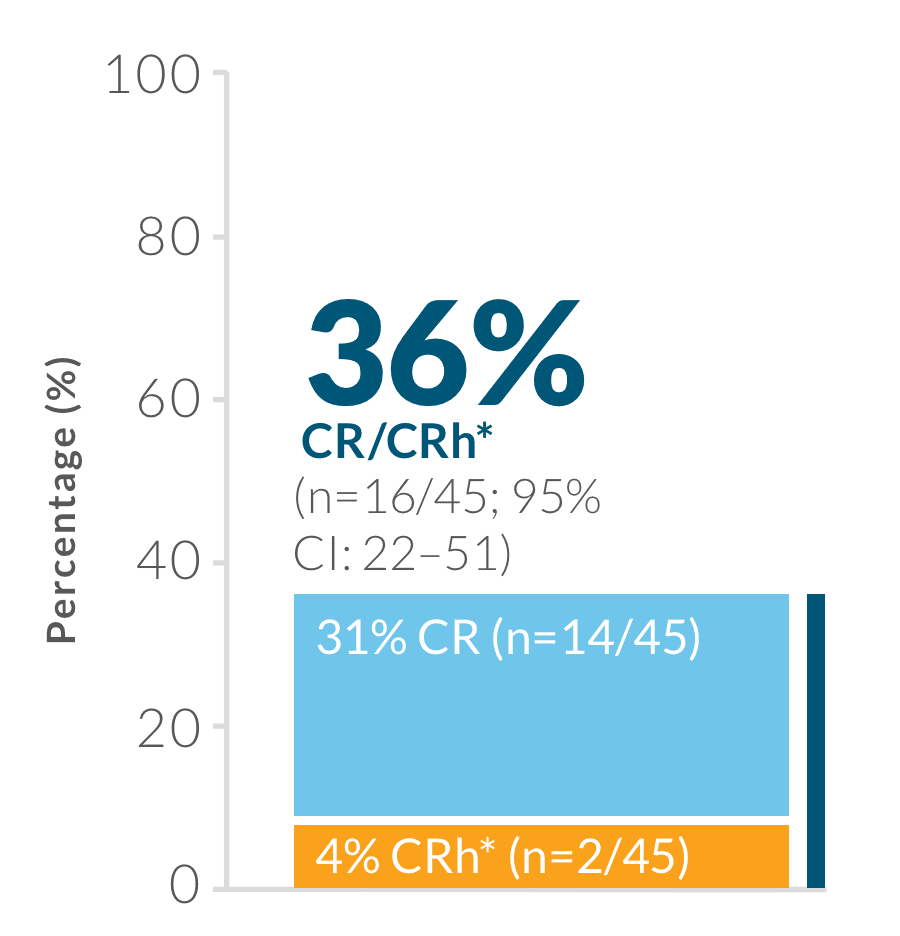
CR was defined as ≤ 5% blasts in the BM, no evidence of disease, and full recovery of peripheral blood counts (platelets > 100,000/mcL and ANC > 1,000/mcL).1
CRh* was defined as ≤ 5% blasts in the BM, no evidence of disease, and partial recovery of peripheral blood counts (platelets > 50,000/mcL and ANC > 500/mcL).1
Consistent response across subgroups1,19
40%
(n=4/10)
CR among patients with T315I mutation†
47%
(n=8/17)
CR/CRh* rate among
patients treated with
≥ 3 prior TKIs†
35%
(n=8/23)
CR/CRh* rate among patients who had received prior ponatinib therapy‡
†CR/CRh* in these subgroups was a prespecified analysis in ALCANTARA; however, the CR/CRh* efficacy in these subgroups was not a study objective and the study was not powered to assess CR/CRh* efficacy in these subgroups.20
‡Response in this subgroup is a post hoc analysis in ALCANTARA, thus the efficacy in this subgroup was not a study objective and the study was not powered to assess efficacy in this subgroup.20
ALL, acute lymphoblastic leukemia; ANC, absolute neutrophil count; BM, bone marrow; CI, confidence interval; CR, complete remission; CRh*, complete remission with partial hematologic recovery; HSCT, allogeneic hematopoietic stem cell transplantation; MRD, measurable or minimal residual disease; NCCN, National Comprehensive Cancer Network; OS, overall survival; Ph(+), Philadelphia chromosome–positive; RFS, relapse-free survival; R/R, relapsed or refractory; TKI, tyrosine kinase inhibitor.
88%
(n=14/16)
of patients with CR/CRh* were MRD negative
within
the first 2 treatment cycles1,19
PCR, polymerase chain reaction.
ALCANTARA study design: BLINCYTO® single-agent immunotherapy was evaluated in an open-label, single-arm, multicenter phase 2 study (N=45) in adult patients with Ph(+) R/R B-cell precursor ALL who progressed after, or were intolerant to second- or later-generation TKI therapy and were intolerant or refractory to imatinib.1,19
BLINCYTO® single-agent immunotherapy19
| Key inclusion criteria19 |
|---|
| Patients ≥ 18 years of age |
| Relapsed after or refractory to at least 1 second- or later-generation TKI or intolerant to second- or later-generation TKI and intolerant or refractory to imatinib |
| > 5% BM blasts |
| ECOG performance status ≤ 2 |
| Key exclusion criteria19 |
| HSCT within 12 weeks |
| Active acute or chronic Grade 2 to 4 GvHD |
| Systemic treatment of GvHD within 2 weeks before treatment start |
| History or presence of clinically relevant CNS pathology |
| Active CNS ALL |
| Isolated extramedullary disease |
Baseline demographic and disease characteristics (N=45)1,19
| Sex, n (%) | |
|---|---|
| Male | 24 (53) |
| Age group, n (%) | |
| 18 to < 55 years | 22 (49) |
| 55 to < 65 years | 11 (24) |
| ≥ 65 years | 12 (27) |
| Prior TKI exposure, n (%)† | |
| Dasatinib | 39 (87) |
| Imatinib‡ | 25 (56) |
| Ponatinib | 23 (51) |
| Nilotinib | 16 (36) |
| T315I mutation, n (%) | 10 (27)§ |
| Prior HSCT, n (%) | 20 (44) |
| ≥ 2 prior TKI treatments, n (%) | 38 (84) |
| ≥ 3 prior TKI treatments, n (%) | 17 (38) |
†Prior TKI use was not mutually exclusive.19
‡One patient was resistant to imatinib and was never exposed to a second-generation or later TKI (protocol deviation).19
§Of 37 patients evaluable for TKI mutational analysis.19
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; GvHD, graft versus host disease; IV, intravenous.
NCCN Guidelines recommend blinatumomab (BLINCYTO®) for adult patients with Ph(+) R/R B-cell precursor ALL7
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
BLINCYTO® is contraindicated in patients with a known hypersensitivity to blinatumomab or to any component of the product formulation.
The incidence of signs and symptoms consistent with ICANS in clinical trials was 7.5%. The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS. Monitor patients for signs or symptoms of neurological toxicities, including ICANS, and interrupt or discontinue BLINCYTO® as outlined in the PI.
Use the preservative-free preparations of BLINCYTO® where possible in neonates. When prescribing BLINCYTO® (with preservative) for neonatal patients, consider the combined daily metabolic load of benzyl alcohol from all sources including BLINCYTO® (with preservative), other products containing benzyl alcohol or other excipients (e.g., ethanol, propylene glycol) which compete with benzyl alcohol for the same metabolic pathway.
Monitor neonatal patients receiving BLINCYTO® (with preservative) for new or worsening metabolic acidosis. The minimum amount of benzyl
alcohol at which serious adverse reactions may occur in neonates is not known. The BLINCYTO® 7-Day bag (with preservative) contains 7.4 mg of benzyl alcohol per mL.
Please see BLINCYTO® full Prescribing Information, including BOXED WARNINGS.
BLINCYTO® is a registered trademark of Amgen Inc.
WARNING: CYTOKINE RELEASE SYNDROME and
NEUROLOGICAL TOXICITIES including IMMUNE EFFECTOR CELL-ASSOCIATED
NEUROTOXICITY SYNDROME