CR/CRh*/
CRi rates of
44%
(n=119/271)
(95% CI: 37.9–50.0)
BLINCYTO® (blinatumomab) is indicated for the treatment of CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL) in adult and pediatric patients one month and older with:
Philadelphia chromosome-negative disease in the consolidation phase of multiphase chemotherapy ... Read More
The content of the linked site is the sole responsibility of the site provider. Amgen Inc. does not control or endorse this third-party website.


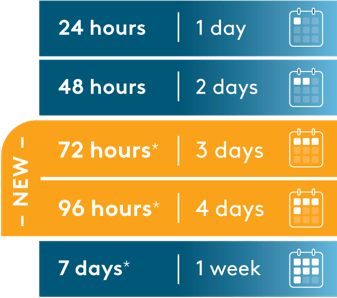
The choice between these infusion duration options should be made by the treating healthcare professional, considering the frequency of the infusion bag changes and the weight of the patient.†
*Prepared with Bacteriostatic 0.9% Sodium Chloride Injection (containing 0.9% benzyl alcohol).
†Use the preservative-free preparations of BLINCYTO® where possible in neonates. When prescribing BLINCYTO® (with preservative) for neonatal patients, consider the combined daily metabolic load of benzyl alcohol from all sources including BLINCYTO® (with preservative), other products containing benzyl alcohol or other excipients (eg, ethanol, propylene glycol) which compete with benzyl alcohol for the same metabolic pathway. Serious adverse reactions, including fatal reactions and the “gasping syndrome,” have been reported in very low birth weight neonates born weighing less than 1500 g, and early preterm neonates who received intravenous drugs containing benzyl alcohol as a preservative.
Please see Indications in above "Indications" tray and full Important Safety Information, including BOXED WARNINGS, below.
Reference: BLINCYTO® (blinatumomab) prescribing information, Amgen.

R/R: The TOWER landmark study


WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
BLINCYTO® is contraindicated in patients with a known hypersensitivity to blinatumomab or to any component of the product formulation.
Monitor patients for signs or symptoms of these events. If severe CRS occurs, interrupt BLINCYTO® until CRS resolves. Discontinue BLINCYTO® permanently if life-threatening CRS occurs. Administer corticosteroids for severe or life-threatening CRS.
The incidence of signs and symptoms consistent with ICANS in clinical trials was 7.5%. The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS. There is limited experience with BLINCYTO® in patients with active ALL in the central nervous system (CNS) or a history of neurologic events. Patients with a history or presence of clinically relevant CNS pathology were excluded from clinical studies. Patients with Down Syndrome may have a higher risk of seizures with BLINCYTO® therapy; consider seizure prophylaxis prior to initiation of BLINCYTO for these patients.
Monitor patients for signs and symptoms of neurological toxicities, including ICANS, and interrupt or discontinue BLINCYTO® and/or treat with corticosteroids as outlined in the PI. Advise outpatients to contact their healthcare professional if they develop signs or symptoms of neurological toxicities.
Use the preservative-free preparations of BLINCYTO® where possible in neonates. When prescribing BLINCYTO® (with preservative) for neonatal patients, consider the combined daily metabolic load of benzyl alcohol from all sources including BLINCYTO® (with preservative), other products containing benzyl alcohol or other excipients (e.g., ethanol, propylene glycol) which compete with benzyl alcohol for the same metabolic pathway.
Monitor neonatal patients receiving BLINCYTO® (with preservative) for new or worsening metabolic acidosis. The minimum amount of benzyl alcohol at which serious adverse reactions may occur in neonates is not known. The BLINCYTO® 72-Hour bag (with preservative) and 96-Hour bag (with preservative) contain 2.5 mg of benzyl alcohol per mL, and the 7-Day bag (with preservative) contains 7.4 mg of benzyl alcohol per mL
BLINCYTO® (blinatumomab) is indicated for the treatment of CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL) in adult and pediatric patients one month and older with:
Please see BLINCYTO® full Prescribing Information, including BOXED WARNINGS.
BLINCYTO® is a registered trademark of Amgen Inc.
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
References: 1. BLINCYTO® (blinatumomab) prescribing information, Amgen. 2. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836-847. 3. Dombret H, Topp MS, Schuh A, et al. Blinatumomab versus chemotherapy in first salvage or in later salvage for B-cell precursor acute lymphoblastic leukemia. Leuk Lymphoma. 2019;60:2214-2222. 4. Data on file, Amgen; 2014. 5. Rambaldi A, Huguet F, Zak P, et al. Maintenance therapy with blinatumomab in adults with relapsed/refractory B-precursor acute lymphoblastic leukemia: overall survival in adults enrolled in a phase 3 open-label trial. Presented at: 59th ASH Annual Meeting and Exposition; December 9–12, 2017; Atlanta, GA. Abstract 2552. 6. Topp MS, Zimmerman Z, Cannell P, et al. Health-related quality of life (HRQoL) of blinatumomab versus standard of care (SOC) chemotherapy in patients with relapsed or refractory Philadelphia negative B-cell precursor acute lymphoblastic leukemia in a randomized, open-label phase 3 study (TOWER). Blood. 2016;128:222. 7. Data on file, Amgen; 2017. 8. Data on file, Amgen; 2016. 9. European Organisation for Research and Treatment of Cancer. EORTC QLQ-C30 (version 3). https://www.eortc.org/app/uploads/sites/2/2018/08/Specimen-QLQ-C30-English.pdf. Accessed April 2, 2025. 10. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(suppl):836-847. 11. Kantarjian H, DeAngelo D, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375:740-753. 12. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Lymphoblastic Leukemia V.2.2025. ©National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed August 4, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.