81%
(n=70/86)
BLINCYTO® (blinatumomab) is indicated for the treatment of CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL) in adult and pediatric patients one month and older with:
Philadelphia chromosome-negative disease in the consolidation phase of multiphase chemotherapy ... Read More
The content of the linked site is the sole responsibility of the site provider. Amgen Inc. does not control or endorse this third-party website.



The choice between these infusion duration options should be made by the treating healthcare professional, considering the frequency of the infusion bag changes and the weight of the patient.†
*Prepared with Bacteriostatic 0.9% Sodium Chloride Injection (containing 0.9% benzyl alcohol).
†Use the preservative-free preparations of BLINCYTO® where possible in neonates. When prescribing BLINCYTO® (with preservative) for neonatal patients, consider the combined daily metabolic load of benzyl alcohol from all sources including BLINCYTO® (with preservative), other products containing benzyl alcohol or other excipients (eg, ethanol, propylene glycol) which compete with benzyl alcohol for the same metabolic pathway. Serious adverse reactions, including fatal reactions and the “gasping syndrome,” have been reported in very low birth weight neonates born weighing less than 1500 g, and early preterm neonates who received intravenous drugs containing benzyl alcohol as a preservative.
Please see Indications in above "Indications" tray and full Important Safety Information, including BOXED WARNINGS, below.
Reference: BLINCYTO® (blinatumomab) prescribing information, Amgen.

Study design: A multinational, randomized, controlled, phase 3 trial of BLINCYTO® alternating with chemotherapy vs chemotherapy alone in frontline consolidation in 224 newly diagnosed patients aged 30–70 years with Ph(–) B-cell precursor ALL. OS was calculated from time of randomization until death due to any cause.1,2 3-year KM estimates for OS (primary endpoint) were 84.8% in the BLINCYTO® arm (n=112) vs 69.0% in the chemotherapy only arm (n=112); HR: 0.42 (95% CI: 0.24–0.75);* P = 0.003 (the stratified log-rank test). Median follow-up was 3.6 years in both arms.1 5-year KM estimates for OS were 82.4% in the BLINCYTO® arm (n=112) vs 62.5% in the chemotherapy only arm (n=112); HR: 0.44 (95% Cl: 0.25–0.76).1,†
*The hazard ratio estimates are obtained from a stratified Cox regression model at the third interim analysis.1
†In a later analysis with a median follow-up of 4.5 years.1
OS in patients who were MRD(–)4
This was an unplanned landmark analysis of the E1910 study. Efficacy in this subgroup was not a prespecified study objective and the study was not powered to assess efficacy in this subgroup.4
E1910 study design: A multinational, randomized, controlled, phase 3 trial that compared BLINCYTO® alternating with chemotherapy with chemotherapy alone in frontline consolidation in 224 newly diagnosed patients aged 30–70 years with Ph(–) B-cell precursor ALL.1,2
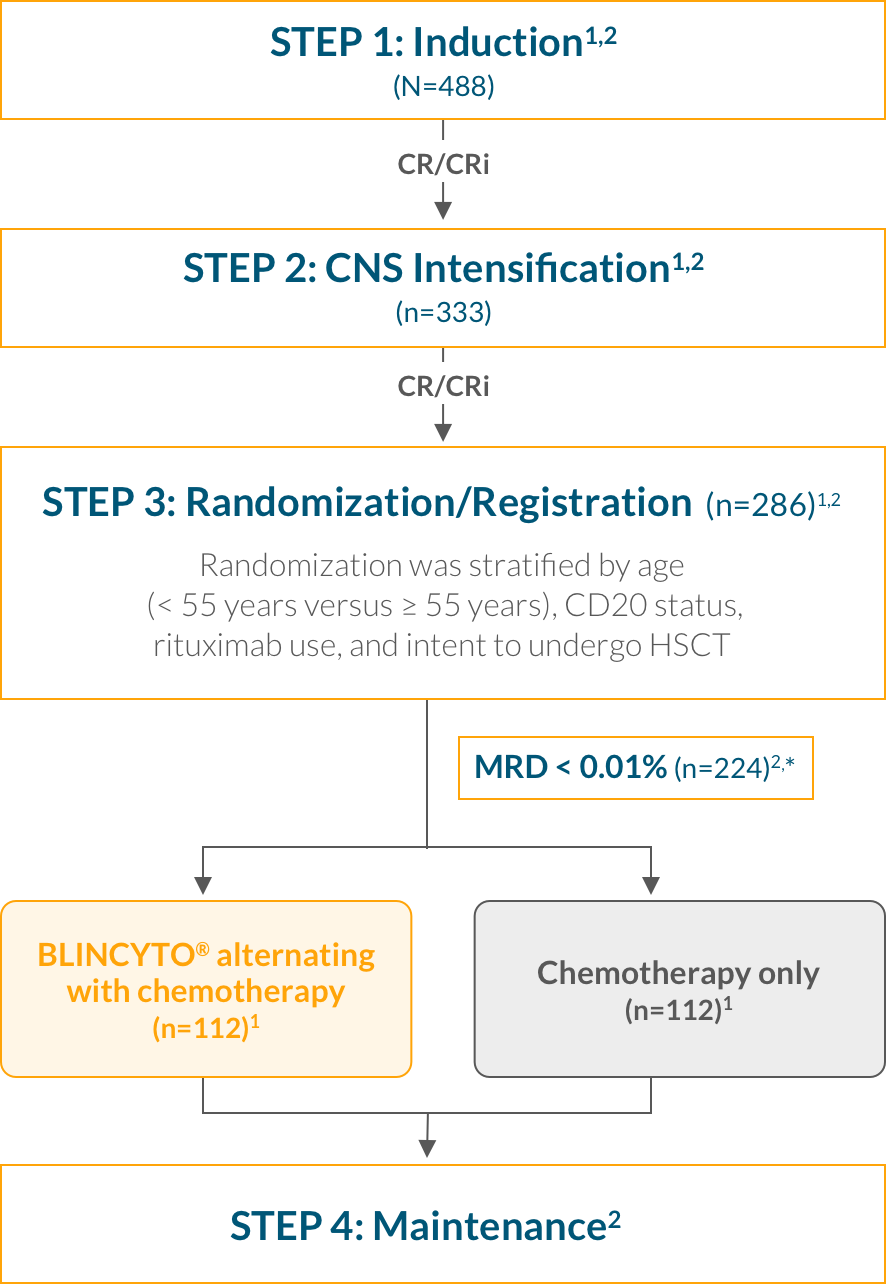
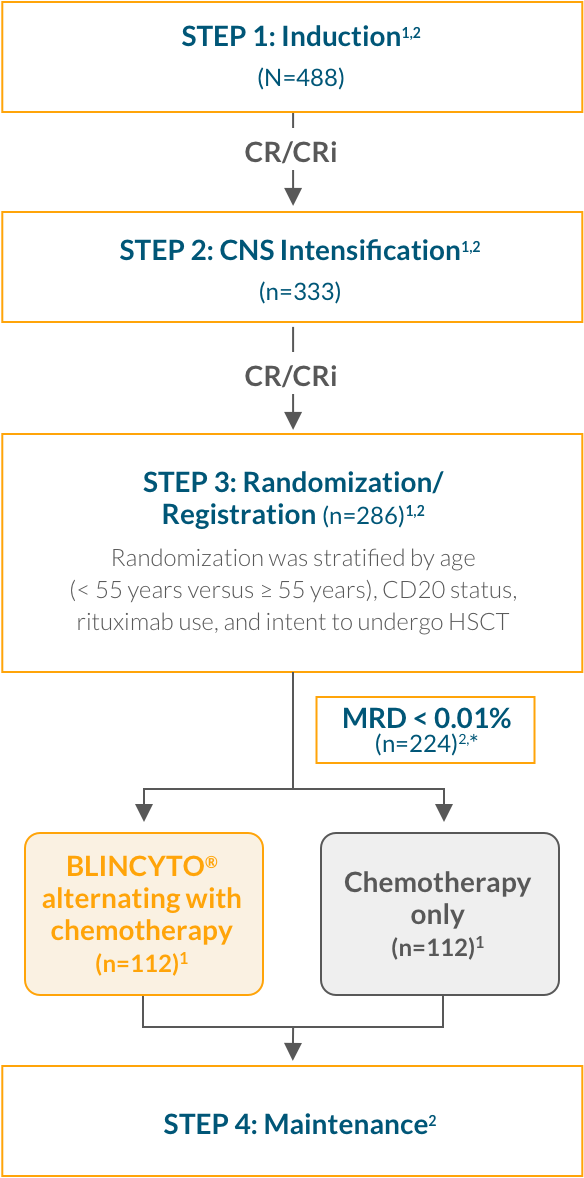
Step 1: Induction—Patients received 2.5 months of a BFM-type induction regimen (modified from the E2993/UKALLXII protocol)2
Step 2: CNS Intensification—Patients who achieved CR/CRi then received intensification therapy with high-dose methotrexate and pegaspargase2
Step 3: Randomization—After intensification therapy, patients who were still in CR/CRi were randomized to receive 4 cycles of consolidation chemotherapy with or without up to 4 cycles of BLINCYTO® 1,2
Step 4: Maintenance—Patients received POMP therapy through 2.5 years from start of intensification among patients who completed consolidation but did not go to HSCT1,2
| Key inclusion criteria1,8 |
|---|
| Patients aged 30–70 years |
| Newly diagnosed Ph(–) B-cell precursor ALL |
| ECOG PS 0–2§ |
| Key exclusion criteria8 |
| Mature B-ALL (Burkitt's-like leukemia) |
| Receiving treatment for concurrent active malignancy |
| History or presence of clinically relevant CNS pathology |
§Step 3 (randomization) inclusion criterion.8
| Characteristic |
BLINCYTO® + chemotherapy arm (n=112) |
Chemotherapy
only arm (n=112) |
||
| Age | ||||
| Median, years (min, max) | 52 (31, 69) | 50 (30, 70) | ||
| Sex, n (%) | ||||
| Male | 55 (49) | 56 (50) | ||
| Race, n (%) | ||||
| American Indian or Alaska Native | 2 (2) | 1 (1) | ||
| Asian | 3 (3) | 2 (2) | ||
| Black (or African American) | 9 (8) | 4 (4) | ||
| Native Hawaiian or Other Pacific Islander | 1 (1) | 0 | ||
| White | 87 (78) | 89 (79) | ||
| Not reported | 5 (4) | 6 (5) | ||
| Unknown | 5 (4) | 10 (9) | ||
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 13 (12) | 10 (9) | ||
| Not Hispanic or Latino | 95 (85) | 95 (85) | ||
| Not reported | 1 (1) | 2 (2) | ||
| Unknown | 3 (3) | 5 (4) | ||
| Stratification Factors, n (%) | ||||
| Age < 55 years at randomization | 65 (58) | 65 (58) | ||
| CD20-positive | 45 (40) | 46 (41) | ||
| Rituximab use | 33 (29) | 36 (32) | ||
| Planned HSCT | 36 (32) | 35 (31) | ||
Blinatumomab is an NCCN Guidelines®–recommended treatment option in consolidation for both AYA and adult patients with Ph(–) B-cell precursor ALL regardless of MRD status.3
Study design: N=86, an open-label, single-arm phase 2 study of adult patients with MRD(+) B-cell precursor ALL who had received at least 3 chemotherapy blocks of standard ALL therapy, were in hematologic complete remission (defined as < 5% blasts in bone marrow, absolute neutrophil count > 1 Gi/L, platelets > 100 Gi/L), and had MRD at a level of ≥ 0.1% using an assay with a minimum sensitivity of 0.01%. Primary endpoint: 81% (n=70/86) of patients had no detectable MRD assessed after 1 treatment cycle with BLINCYTO®.* Select secondary endpoints: OS, hematologic RFS at 18 months, duration of hematologic remission.1,9
*Defined as the absence of detectable MRD confirmed in an assay with minimum sensitivity of 0.01% for 6 patients and ≤ 0.005% for 80 patients. Undetectable MRD was achieved by 65 of 80 patients with an assay sensitivity of at least 0.005%.1
Primary endpoint: complete MRD response1,*
81%
(n=70/86)
of patients had no detectable MRD†
| Patient response | n | Median OS | 95% CI |
| MRD responders at cycle 1 | 84 | NR | 29.5–NR |
| MRD nonresponders at cycle 1 | 23 | 14.4 months | 3.8–32.3 |
Adults ≥ 18 years of age with B‑cell precursor ALL in hematologic first complete remission or second complete remission with MRD ≥ 0.1%*
Follow-up:
30 days
(safety);
24 months
plus
5-year survival follow-up
| Key inclusion criteria1 |
|---|
| Adults ≥ 18 years of age with B-cell precursor ALL in hematologic complete remission |
| Measurable residual disease (MRD)‡ level of ≥ 0.1% (molecular relapse or molecular failure)§ |
| < 5% blasts in bone marrow |
| ANC > 1 Gi/L |
| Platelets > 100 Gi/L |
| Key exclusion criteria11 |
| Prior HSCT |
| Presence of circulating blasts or current extramedullary disease |
| History of relevant CNS pathology or current relevant CNS pathology |
| Prior systemic cancer chemotherapy within 2 weeks or radiotherapy within 4 weeks |
| Baseline characteristics of patients (N=86) | |
|---|---|
| Age | |
| Median, years (min, max) | 43 (18, 76) |
| ≥ 65 years, n (%) | 10 (12) |
| Males, n (%) | 50 (58) |
| Philadelphia chromosome disease status, n (%) | |
| Positive | 1 (1) |
| Negative | 85 (99) |
| Relapse history, n (%) | |
| In first complete remission | 61 (71) |
| In second complete remission | 25 (29) |
| Baseline MRD levels,* n (%) | |
| ≥ 10% | 7 (8) |
| ≥ 1% and < 10% | 34 (40) |
| ≥ 0.1% and < 1% | 45 (52) |
Blinatumomab is recommended by NCCN as a preferred therapy option in consolidation for AYA and adult patients with Ph(–) B-cell precursor ALL who have persistent or rising MRD and have not previously received blinatumomab; the NCCN Guidelines recommend “therapy aimed at eliminating MRD prior to allogeneic HCT,” when possible.3
Study design: A large (N=405), international, randomized, controlled, phase 3 study of single-agent BLINCYTO® vs SOC chemotherapy in patients ≥ 18 years of age: refractory to primary induction therapy or to last therapy, in first relapse (first remission duration < 12 months), in second or later relapse, or in any relapse after HSCT. Primary endpoint was mOS: 7.7 months for BLINCYTO® (n=271) vs 4.0 months for SOC chemotherapy (n=134); P = 0.012; HR: 0.71 (95% Cl: 0.55–0.93).1 Selected secondary endpoints: CR within 12 weeks after initiation of treatment, CR/CRh*/CRi within 12 weeks after initiation of treatment, MRD remission rate, duration of remission, adverse event rates.1,14
BLINCYTO® is an established approach that significantly improved survival vs SOC chemotherapy1
CI, confidence interval; CR, complete remission; CRh*, complete remission with partial hematologic recovery; CRi, complete remission with incomplete hematologic recovery; HR, hazard ratio; HSCT, allogeneic hematopoietic stem cell transplantation; mOS, median overall survival; SOC, standard-of-care.
*OS in patients treated in first salvage was a prespecified subgroup analysis in TOWER; however, the OS efficacy in this subgroup was not a study objective and the study was not powered to assess OS efficacy in this subgroup.16
NR, not reached; OS, overall survival.
*OS in patients censored for allogeneic transplant was a prespecified sensitivity subgroup analysis in TOWER; however, the OS efficacy in this subgroup was not a study objective, and the study was not powered to assess OS efficacy in this subgroup.16
CR/CRh*/
CRi rates of
44%
(n=119/271)
(95% CI: 37.9–50.0)
for patients treated with BLINCYTO® vs 25% (n=33/134) (95% CI: 17.6–32.8) for patients treated with SOC chemotherapy14 (P < 0.001)
Median duration of remission for patients who achieved CR/CRh*/CRi14
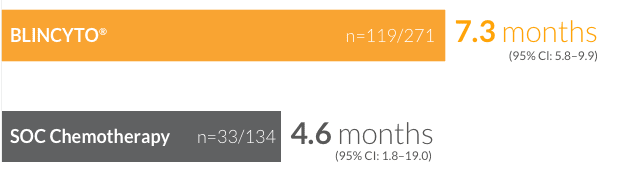
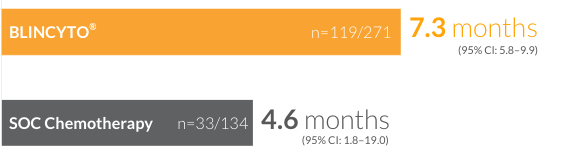
†CR was defined as ≤ 5% blasts in the BM, no evidence of disease, and full recovery of peripheral blood counts (platelets > 100,000/μL and ANC > 1,000/μL). CRh* was defined as ≤ 5% blasts in the BM, no evidence of disease, and partial recovery of peripheral blood counts (platelets > 50,000/μL and ANC > 500/μL). CRi was defined as ≤ 5% blasts in the BM, no evidence of disease, and incomplete recovery of peripheral blood counts (platelets > 100,000/μL or ANC > 1,000/μL).14
ANC, absolute neutrophil count; BM, bone marrow.
74%
(n=20/27)
of patients achieved a best response of complete remission during continued therapy
BLINCYTO®
76%
(n=90/119)
vs SOC chemotherapy (n=16/33): 48%
†Molecular remission was assessed in patients achieving CR/CRh*/CRi, and was defined as MRD by PCR or flow cytometry with a minimum assay sensitivity of14 < 1 x 10–4.
AE, adverse event; PCR, polymerase chain reaction.
Mean changes in HRQoL functional domains and symptoms at the end of cycle 1 (day 29)18
Click here for the EORTC QoL Questionnaire from the TOWER Study21
*The PRO instrument—The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30)—is a validated self-rating questionnaire used to assess patients’ perceptions of treatment effectiveness in oncology.19
HRQoL, health-related quality of life; PRO, patient-reported outcome; QoL, quality of life.
Prephase treatment:
Premedication:
Primary endpoint:14
Select secondary endpoints:14
| Key inclusion criteria1,14 |
|---|
| Patients ≥ 18 years of age |
| Ph(–) R/R B-cell precursor ALL occurring as refractory to primary induction therapy or to last therapy, or untreated first relapse (first remission duration < 12 months), or in second or later relapse, or in any relapse after HSCT |
| With > 5% blasts in the BM and ECOG PS ≤ 2 |
| Key exclusion criteria14 |
| Other active cancers |
| Clinically relevant pathologic condition of the CNS |
| Isolated extramedullary disease |
| Autoimmune disease |
| Acute GvHD of Grade ≥ 2, active chronic GvHD |
| Allogeneic stem cell transplantation within 12 weeks before randomization |
| Autologous stem cell transplantation within 6 weeks before randomization |
| Chemotherapy or radiotherapy within 2 weeks before randomization |
| Use of immunotherapy within 4 weeks before randomization |
| Ongoing use of investigational treatment |
BLINCYTO® was studied in a wide range of adult patients, including those with a poor prognosis14
|
BLINCYTO® (N=271) |
SOC Chemotherapy (N=134) | |
| Age | ||
| Mean ± SD, years | 41 ± 17 | 41 ± 17 |
| Range, years | 18–80 | 18–78 |
| Study entry criteria, n (%) | ||
| Refractory to primary or salvage therapy | 115 (42) | 54 (40) |
| In early first relapse (CR1 duration < 12 months) | 76 (28) | 37 (28) |
| In untreated second or later relapse§ | 32 (12) | 16 (12) |
| Relapsed after HSCT§ | 46 (17) | 27 (20) |
| Not specified | 2 (1) | 0 |
| Prior salvage therapy, n (%) | 171 (63) | 70 (52) |
| Prior transplant, n (%) | ||
| Yes | 94 (35) | 46 (34) |
| No | 176 (65) | 87 (65) |
| Unknown | 1 (0) | 1 (1) |
| Disease burden, n (%) | ||
| ≥ 50% BM blasts | 201 (74) | 104 (78) |
Blinatumomab is an NCCN Category 1 recommended therapy option for both AYA and adult patients with Ph(–) R/R B-cell precursor ALL3
In a real-world evidence study, the observed efficacy of BLINCYTO® was reflective of the TOWER clinical study data1,14,24,*
*Five patients who could not titrate to the full dose due to tolerance were included in the analysis.24
ALL, acute lymphoblastic leukemia; ANC, absolute neutrophil count; BM, bone marrow; CR, complete remission; CRi, complete remission with incomplete hematologic recovery; DOR, duration of response; HSCT, allogeneic hematopoietic stem cell transplantation; MRD, measurable or minimal residual disease; OS, overall survival; Ph(–), Philadelphia chromosome–negative; Ph(+), Philadelphia chromosome–positive; R/R, relapsed or refractory; TKI, tyrosine kinase inhibitor.
Median OS24
9.2 months
(n=158)
*One patient did not have data available for the survival analysis and is not included in the survival analyses. However, note that this patient is included in the toxicity and response evaluations.24
†Estimated from the time of BLINCYTO® treatment initiation to death or lost to follow-up.24
CR/CRi
rates of24
57%
(n=86/151)
for patients on
BLINCYTO®
Median DOR for patients who achieved CR/CRi24,‡
for patients treated with BLINCYTO®
(n=86)
Duration of response by tumor burden24


*The exact assessment timing was not specified for this endpoint.24
†Data were missing for 8 patients in this analysis.24
‡Adjusted for HSCT.24
MRD response24
78%
(n=65/83)
for patients
in CR treated
with
BLINCYTO®
Proceeded to HSCT24
for all patients treated with BLINCYTO®
(n=71/159)
*The exact assessment timing was not specified for this endpoint.24
Baseline characteristics (n=159)24
| Age* | |
|---|---|
| Mean ± SD, years | 45 ± 17 |
| Range, years | 8–79 |
| Study entry criteria, n (%) | |
| Refractory to primary therapy | 41 (26.6) |
| < 18 months to first progression | 76 (49.4) |
| Cytogenetic abnormalities, n (%) | |
| No | 62 (39.0) |
| Yes | 97 (61.0) |
| ≥ 3 previous therapies, n (%) | 36 (22.8) |
| Prior transplant, n (%) | |
| Yes | 27 (17.0) |
| No | 132 (83.0) |
| BM blasts at initiation of BLINCYTO®, n (%) | |
| BM blasts ≥ 50%, n (%) | 57 (45.2) |
NR, not reached; PCR, polymerase chain reaction; SD, standard deviation.
Study design: BLINCYTO® single-agent immunotherapy was evaluated in an open-label, single-arm, multicenter phase 2 study (N=45) in adult patients with Ph(+) R/R B-cell precursor ALL who progressed after, or were intolerant to second- or later-generation TKI therapy and were intolerant or refractory to imatinib. Primary endpoint was CR/CRh* rate within the first 2 treatment cycles: 36% (n=16/45; 95% CI: 22–51).1,25 Selected secondary endpoints: MRD response rate during the first two cycles of treatment, RFS, OS, HSCT after BLINCYTO®-induced remission.1,25
BLINCYTO® is an effective treatment for Ph(+) B-cell precursor ALL1
Primary endpoint: CR/CRh* rate within the first 2 treatment cycles1,25

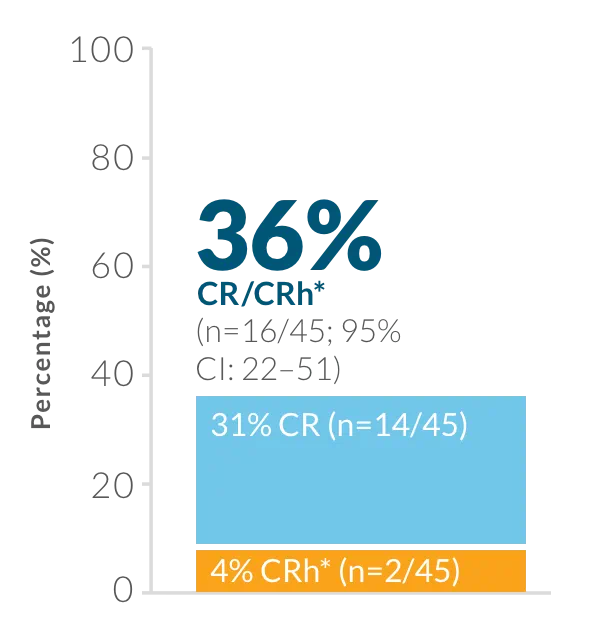
CR was defined as ≤ 5% blasts in the BM, no evidence of disease, and full recovery of peripheral blood counts (platelets > 100,000/mcL and ANC > 1,000/mcL).1
CRh* was defined as ≤ 5% blasts in the BM, no evidence of disease, and partial recovery of peripheral blood counts (platelets > 50,000/mcL and ANC > 500/mcL).1
Consistent response across subgroups1,25
40%
(n=4/10)
CR among patients with T315I mutation†
47%
(n=8/17)
CR/CRh* rate among
patients treated with
≥ 3 prior TKIs†
35%
(n=8/23)
CR/CRh* rate among patients who had received prior ponatinib therapy‡
†CR/CRh* in these subgroups was a prespecified analysis in ALCANTARA; however, the CR/CRh* efficacy in these subgroups was not a study objective and the study was not powered to assess CR/CRh* efficacy in these subgroups.26
‡Response in this subgroup is a post hoc analysis in ALCANTARA, thus the efficacy in this subgroup was not a study objective and the study was not powered to assess efficacy in this subgroup.26
ALL, acute lymphoblastic leukemia; ANC, absolute neutrophil count; BM, bone marrow; CI, confidence interval; CR, complete remission; CRh*, complete remission with partial hematologic recovery; HSCT, allogeneic hematopoietic stem cell transplantation; MRD, measurable or minimal residual disease; OS, overall survival; Ph(+), Philadelphia chromosome–positive; RFS, relapse-free survival; R/R, relapsed or refractory; TKI, tyrosine kinase inhibitor.
88%
(n=14/16)
of patients with CR/CRh* were MRD negative
within
the first 2 treatment cycles1,25
PCR, polymerase chain reaction.
ALCANTARA study design: BLINCYTO® single-agent immunotherapy was evaluated in an open-label, single-arm, multicenter phase 2 study (N=45) in adult patients with Ph(+) R/R B-cell precursor ALL who progressed after, or were intolerant to second- or later-generation TKI therapy and were intolerant or refractory to imatinib.1,25
BLINCYTO® single-agent immunotherapy
| Key inclusion criteria25 |
|---|
| Patients ≥ 18 years of age |
| Relapsed after or refractory to at least 1 second- or later-generation TKI or intolerant to second- or later-generation TKI and intolerant or refractory to imatinib |
| > 5% BM blasts |
| ECOG performance status ≤ 2 |
| Key exclusion criteria25 |
| HSCT within 12 weeks |
| Active acute or chronic Grade 2 to 4 GvHD |
| Systemic treatment of GvHD within 2 weeks before treatment start |
| History or presence of clinically relevant CNS pathology |
| Active CNS ALL |
| Isolated extramedullary disease |
Baseline demographic and disease characteristics (N=45)1,25
| Sex, n (%) | |
|---|---|
| Male | 24 (53) |
| Age group, n (%) | |
| 18 to < 55 years | 22 (49) |
| 55 to < 65 years | 11 (24) |
| ≥ 65 years | 12 (27) |
| Prior TKI exposure, n (%)† | |
| Dasatinib | 39 (87) |
| Imatinib‡ | 25 (56) |
| Ponatinib | 23 (51) |
| Nilotinib | 16 (36) |
| T315I mutation, n (%) | 10 (27)§ |
| Prior HSCT, n (%) | 20 (44) |
| ≥ 2 prior TKI treatments, n (%) | 38 (84) |
| ≥ 3 prior TKI treatments, n (%) | 17 (38) |
†Prior TKI use was not mutually exclusive.25
‡One patient was resistant to imatinib and was never exposed to a second-generation or later TKI (protocol deviation).25
§Of 37 patients evaluable for TKI mutational analysis.25
AYA, adolescent and young adult; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; GvHD, graft versus host disease; IV, intravenous; NCCN, National Comprehensive Cancer Network.
NCCN Guidelines recommend blinatumomab as a treatment option for both AYA and adult patients with Ph(+) R/R B-cell precursor ALL3
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
BLINCYTO® is contraindicated in patients with a known hypersensitivity to blinatumomab or to any component of the product formulation.
The incidence of signs and symptoms consistent with ICANS in clinical trials was 7.5%. The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS. There is limited experience with BLINCYTO® in patients with active ALL in the central nervous system (CNS) or a history of neurologic events. Patients with a history or presence of clinically relevant CNS pathology were excluded from clinical studies. Patients with Down Syndrome may have a higher risk of seizures with BLINCYTO® therapy; consider seizure prophylaxis prior to initiation of BLINCYTO® for these patients.
Monitor patients for signs and symptoms of neurological toxicities, including ICANS, and interrupt or discontinue BLINCYTO® and/or treat with corticosteroids as outlined in the PI. Advise outpatients to contact their healthcare professional if they develop signs or symptoms of neurological toxicities.
Use the preservative-free preparations of BLINCYTO® where possible in neonates. When prescribing BLINCYTO® (with preservative) for neonatal patients, consider the combined daily metabolic load of benzyl alcohol from all sources including BLINCYTO® (with preservative), other products containing benzyl alcohol or other excipients (e.g., ethanol, propylene glycol) which compete with benzyl alcohol for the same metabolic pathway.
Monitor neonatal patients receiving BLINCYTO® (with preservative) for new or worsening metabolic acidosis. The minimum amount of benzyl alcohol at which serious adverse reactions may occur in neonates is not known. The BLINCYTO® 72-Hour bag (with preservative) and 96-Hour bag (with preservative) contain 2.5 mg of benzyl alcohol per mL, and the 7-Day bag (with preservative) contains 7.4 mg of benzyl alcohol per mL.
BLINCYTO® (blinatumomab) is indicated for the treatment of CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL) in adult and pediatric patients one month and older with:
Please see BLINCYTO® full Prescribing Information, including BOXED WARNINGS.
BLINCYTO® is a registered trademark of Amgen Inc.
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
References: 1. BLINCYTO® (blinatumomab) prescribing information, Amgen. 2. Litzow MR, Sun Z, Mattison RJ, et al. Blinatumomab for MRD-negative acute lymphoblastic leukemia in adults. N Engl J Med. 2024;391:320-333. 3. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Lymphoblastic Leukemia V.3.2024. ©National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed January 2, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 4. Data on file, Amgen; 2024. 5. Food and Drug Administration. https://www.fda.gov/files/drugs/published/Analytical-Procedures-and-Methods-Validation-for-Drugs-and-Biologics.pdf. Accessed January 3, 2025. 6. Data on file, Amgen; [2]; 2024. 7. Luger SM. Sun Z, Mattison RJ, et al. Assessment of outcomes of consolidation therapy by number of cycles of blinatumomab received in newly diagnosed measurable residual disease negative patients with B-lineage acute lymphoblastic leukemia: in the ECOG-ACRIN E1910 randomized phase III National Clinical Trials Network Trial. Poster presented at: 65th ASH Annual Meeting and Exposition; December 9–12, 2023; San Diego, CA. Abstract 2877. 8. ClinicalTrials.gov. Combination chemotherapy with or without blinatumomab in treating patients with newly diagnosed BCR-ABL-negative B lineage acute lymphoblastic leukemia. https://clinicaltrials.gov/study/NCT02003222. Accessed January 3, 2025. 9. Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522-1531. 10. Gökbuget N, Zugmaier G, Dombret H, et al. Curative outcomes following blinatumomab in adults with minimal residual disease B-cell precursor acute lymphoblastic leukemia. Leuk Lymphoma. 2020;61:2665-2673. 11. Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-precursor acute lymphoblastic leukemia. Blood. 2018;131(suppl):1522-1531. 12. Data on file, Amgen; 2018. 13. Food and Drug Administration. BLINCYTO® (blinatumomab) for minimal residual disease positive (MRD+) B-cell precursor acute lymphoblastic leukemia (ALL). https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM603411.pdf. Accessed January 3, 2025. 14. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836-847. 15. Dombret H, Topp MS, Schuh A, et al. Blinatumomab versus chemotherapy in first salvage or in later salvage for B-cell precursor acute lymphoblastic leukemia. Leuk Lymphoma. 2019;60:2214-2222. 16. Data on file, Amgen; 2014. 17. Rambaldi A, Huguet F, Zak P, et al. Maintenance therapy with blinatumomab in adults with relapsed/refractory B-precursor acute lymphoblastic leukemia: overall survival in adults enrolled in a phase 3 open-label trial. Presented at: 59th ASH Annual Meeting and Exposition; December 9–12, 2017; Atlanta, GA. Abstract 2552. 18. Topp MS, Zimmerman Z, Cannell P, et al. Health-related quality of life (HRQoL) of blinatumomab versus standard of care (SOC) chemotherapy in patients with relapsed or refractory Philadelphia negative B-cell precursor acute lymphoblastic leukemia in a randomized, open-label phase 3 study (TOWER). Blood. 2016;128:222. 19. Data on file, Amgen; 2017. 20. Data on file, Amgen; 2016. 21. European Organisation for Research and Treatment of Cancer. EORTC QLQ-C30 (version 3). https://www.eortc.org/app/uploads/sites/2/2018/08/Specimen-QLQ-C30-English.pdf. Accessed January 3, 2025. 22. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(suppl):836-847. 23. Kantarjian H, DeAngelo D, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375:740-753. 24. Data on file, Amgen; 2021. 25. Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome–positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35:1795-1802. 26. Data on file, Amgen; [2]; 2014. 27. ClinicalTrials.gov. Blinatumomab in adults with relapsed/refractory Philadelphia positive B-precursor acute lymphoblastic leukemia. https://clinicaltrials.gov/ct2/show/NCT02000427. Accessed January 3, 2025.